Author: Michele Pittalis
I’m excited to share our latest publication in Chemical Science, a great collaboration with Dominic Shiels and the group of Prof. Ellen Matson at the University of Rochester. This work explores the fascinating chemistry of polyoxometalate (POM) systems and the elusive role of f-electrons in chemical bonding.
Determining the degree of covalent character and f-orbital participation is one of the most fundamental questions in inorganic chemistry. When we study f-block elements like lanthanides and actinides, we always wonder: are their bonds purely ionic, or do the f-orbitals participate in metal-ligand bonding and orbital mixing?
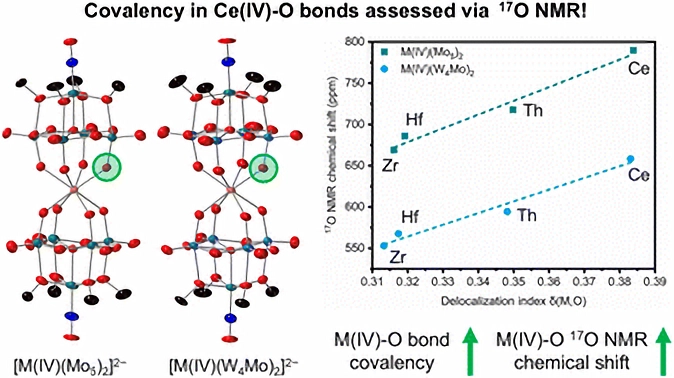
Our collaborators devised a clever synthetic strategy, exploiting the synthetic playground offered by POMs to build a series of “sandwich” complexes with different metal ions: Zr(IV), Hf(IV), Th(IV), and Ce(IV), all having the same exact chemical environment. The isolation of stable, diamagnetic M(IV) complexes allowed them to study this series with ¹⁷O NMR spectroscopy and directly probe the interactions between oxygen atoms and the central metal. By looking at the oxygen chemical shifts, they found that the Ce-O signal was significantly different from the rest, signaling a unique electronic structure.
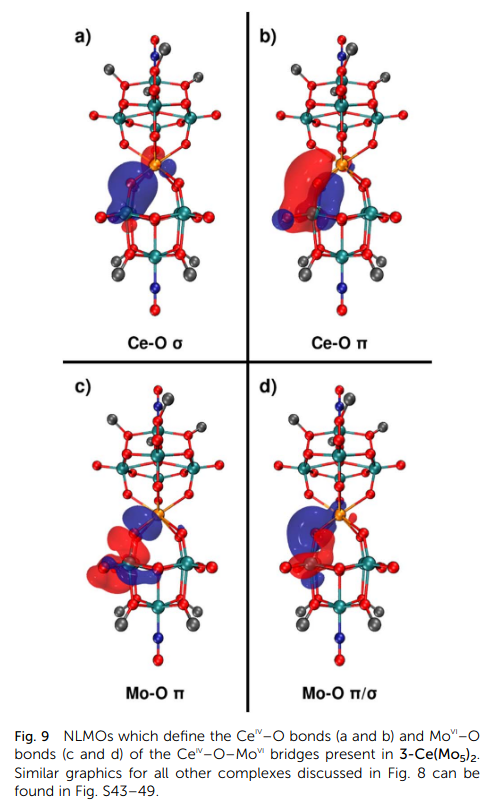
Using density functional theory (DFT), we investigated the electronic origin of this unique spectroscopic signal. Our analysis, using a combination of Natural Bond Order (NBO) and the Quantum Theory of Atoms in Molecules (QTAIM), revealed:
-Compared to the transition elements, the Ce(IV)-O bond possesses an increased covalent character, driven directly by the participation of Ce 4f-electrons.
-In the actinide analogue, Thorium(IV), the 5f-electrons play a smaller role in bonding compared to 6d-electrons, resulting in a lower covalent character than in the cerium complex.
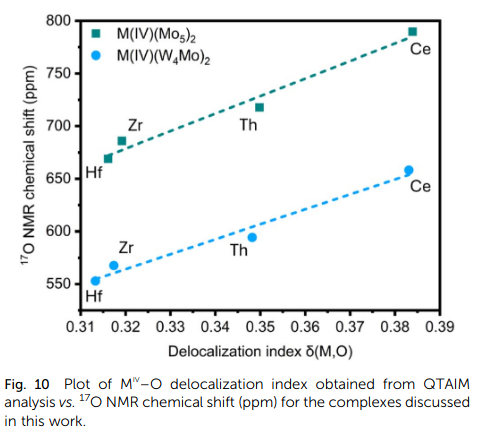
By plotting the calculated Delocalization Index (a QTAIM measure of covalency) against the experimental ¹⁷O NMR chemical shifts, we found an excellent agreement between our computational metrics and the experimental data.
This work is a nice addition to the fascinating chemistry of f-electrons, confirming significant f-orbital participation in the Ce-O bond and highlighting the rich and insightful playground provided by POM systems.